Each cosmetic product must be manufactured in compliance with Good Manufacturing Practices (GMP) and with Article 8 of Cosmetics Regulation EU 1223/2003. These Good Manufacturing Practices are defined in ISO 22716, an international quality standard.
Good Manufacturing Practices (GMP): definition and objectives
Definition of the Good Manufacturing Practices
The Good Manufacturing Practices are a set of guidelines under the ISO 22716 standard that ensure the reproducibility of a cosmetic product and its production quality. These measures must be respected and also apply to the production, control, storage, and shipping procedures for cosmetic products.
GMP compliance of cosmetic products is required by Cosmetics Regulation EC 1223/2003. Additionally, a declaration of compliance with the ISO 22716 standard must be submitted with the Product Information File (PIF).
Note: The GMP only concern the quality of cosmetic products and not employee safety or environmental protection.
What is their purpose?
In the framework of European standard ISO 22716, the Good Manufacturing Practices for cosmetic products have two objectives:
- Verify that the management system of cosmetics facilities complies with the requirements of the European Cosmetics Regulations.
- Ensure cosmetic product access to the European market.
Consequently, applying the GMP ensures consumer product conformity and safety by limiting contamination risks (biological, particulate, and cross).
What activities are covered by the Good Manufacturing Practices?
ISO 22716 sets out a number of standards for quality and reproducibility that cosmetic products must meet if they are to be sold on the European market.
This standard focuses on the production, monitoring, storage and transportation of cosmetics. It also more specifically concerns those in the cosmetics industry who are involved in these activities: finished product manufacturers, distributors, importers, and exporters.
The proper measures described in the ISO 22716 standard for compliance with the GMP concern the following areas:
- Staff
- Premises
- Equipment
- Raw materials and packaging items
- Manufacturing
- Finished Products
- Procedures for products that are off-specification and deviations
- Waste
- Sub-contracting
- Complaints and recalls
- Change management
- Internal audits and quality control
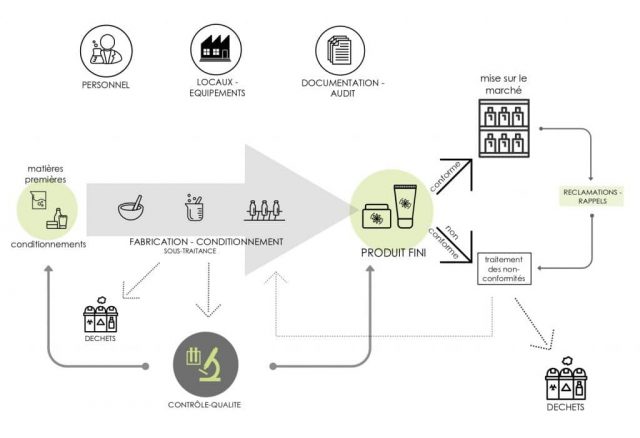
Application Scope of the Good Manufacturing Practices
(Source: ecomundo.eu)
How do we comply with the Good Manufacture Practices?
To obtain a declaration of conformity with the Good Manufacturing Practices, it is necessary to comply with all of the measures listed in the ISO 22716 standard and whose references are published in the OJEU C123/3 of 21 April 2011.
To validate compliance with the ISO 22716 standard, the cosmetics facility must implement an internal audit using a specialised service provider.
The GMP diagnosis and audit may be performed by the responsible person, a third party or an external firm.
Although there is no official certification body for the ISO 22716 standard, the standard is nevertheless mandatory and subject to strict rules.
Once the certificate of compliance has been obtained, it must be added to the cosmetic product’s Product Information File (PIF).
Steps to GMP Compliance:
- : Familiarise yourself with the NF EN ISO 2276 standard in its entirety to fully understand the requirements of the European regulation and ISO standard
- : Evaluate your company’s situation with an internal audit
- : Prepare a procedure relating to product and personnel flows, as well as a set of procedures indicating the rules that apply within the company enabling staff to work in compliance with the GMP
- : Organise your laboratory and manufacturing, reception, storage and shipping areas for GMP compliance
- : Train your personnel in the GMP and ISO standard procedures and requirements
- : Implement the Good Manufacturing Practices for cosmetics by following the procedures created in advance (guarantee of product traceability from receipt of raw materials to delivery of the finished product, management of production and quality control documentation, cleaning of premises and equipment (tanks, pipes, etc.), management of product and personnel flow, training staff in the GMP and procedures according to their position in the company etc.)
- : Monitor these processes and assess their effectiveness via an inspection audit in order to identify possible areas for improvement.

