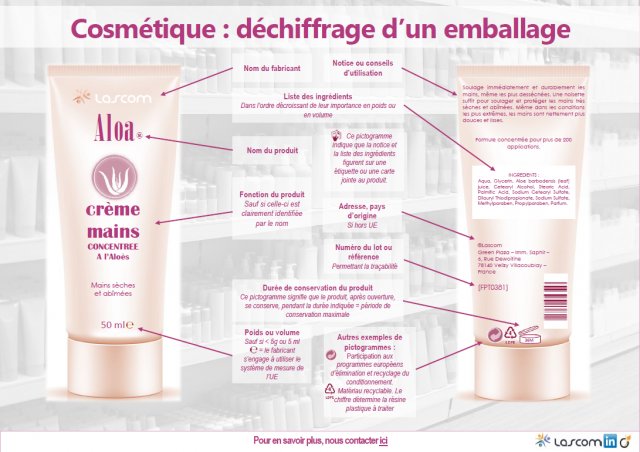
The name (or company name) and address of the Responsible Person
- This information may be provided in abbreviated form as long as the Responsible Person can be clearly identified.
- If multiple addresses are specified, the address at which the PIF (Product Information File) can be accessed must be highlighted (underlined or written in bold, for example).
The cosmetic product’s country of origin
When imported from a country outside the European Union, a product’s labelling must bear the phrase “Made In Name of Country”.
The cosmetic product’s nominal content
This must be stated in grams (gr) or milliliters (ml), with the option of including additional units of measurement (oz).
The DMD (Date of Minimal Durability) and the PAO (Period After Opening)
The DMD
- This is the date until which the cosmetic product continues to fulfil its original function and is safe for human use, provided that it is stored correctly.
- The “hour-glass” symbol indicates the Date of Minimal Durability (DMD) when this is under or equal to 30 months. The DMD is determined by a stability test.
- The date (MM/YYYY or MM/YY or DD/MM/YY) must be stated next to the symbol. In place of the “hour-glass” symbol, the label may alternatively bear the words “Best used before end…”.
The PAO
- The Period After Opening must appear on the product label for all products with a minimum durability of more than 30 months.
- The “open-jar” symbol indicates the Period After Opening (PAO) (or shelf life) when the DMD is greater than 30 months, with the number of months (M) or years (Y) to be indicated inside or beside the open jar symbol. The PAO is jointly determined by a stability and a stimulation test.
Specific precautions for use and warnings
- Depending on the type of product, these may be useful or essential to the consumer.
- Examples: avoid all contact with the eyes, do not apply to wounds, etc.
- If there is insufficient space on the label, the “hand-in-book” symbol should direct the consumer to a leaflet, label, wrapper or card provided with the product which contains more regulatory information.
The batch number or product reference
- This allows for product traceability.
- This information must be displayed on the product directly or on the packaging if the product is too small.
Product’s function
- It is not mandatory to specify this if the function can be clearly identified by the product name.
The list of ingredients
- This list, preceded by the word “ingredients”, must include each of the product’s ingredients, provided in descending order of quantity (weight).
- The ingredients list shall be displayed directly on the packaging, or if there is insufficient space, on a leaflet, wrapper, label or card provided with the product. In this case, the “hand-in-book” symbol should appear on the product to direct the consumer towards this additional information.
